The Science of Perfume Fixatives
Introduction
Ever caught yourself admiring how some fragrances last well into the night, while others disappear after mere minutes? That magical ability to “cling” to skin or clothing is partly thanks to fixatives—an often unsung hero in perfumery.
In simple terms, a fixative slows the evaporation of a perfume’s most volatile notes, ensuring your favorite scent remains enchanting for hours. But how do fixatives actually work, and what’s happening behind the scenes on a chemical level? Let’s explore the fascinating world of perfume fixatives, from classic musks to modern profragrance techniques.
1. Different Paths to Fixation
We often lump all fixatives under one big umbrella, but in reality, there are various ways to “lock” a scent into place. Some rely on creating reversible chemical bonds, while others physically trap fragrance molecules in a specialized matrix. Here are two core strategies:
1.1 True Fixation (Chemical Association)
Schiff Bases
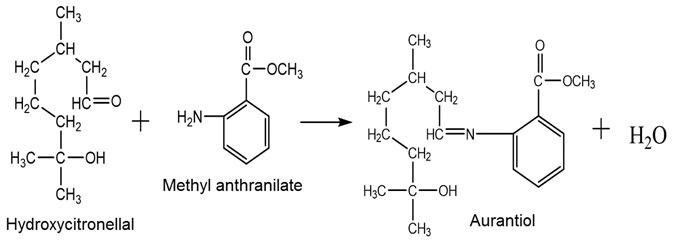
Imagine you have an aldehyde (a key ingredient in many floral scents) and a primary amine (often with a sweet, fruity, or even grape-like aroma). Combine them under the right conditions, and they form what’s known as a Schiff base: a new compound that’s less volatile. In practical perfumery, this means that the aldehyde (the smelliest part) won’t just escape into the air at first spritz. Instead, it slowly separates from the Schiff base over time—giving you that steady trickle of freshness we all crave.
Aurantiol (sometimes called “Schiff 001”) is a classic example, created from hydroxycitronellal (soft floral) and methyl anthranilate (intense grape/floral note).
These bases are especially interesting because you can tweak how fast or slow they “break open” by changing things like pH or the surrounding material, such as gels or even special polymer systems.
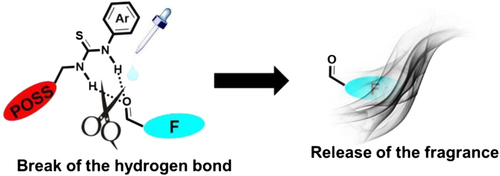
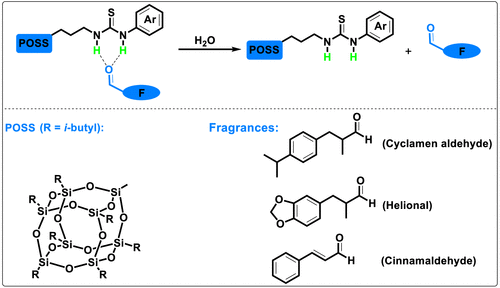
Hydrogen Bond Complexes
Perfume aldehydes can also attach themselves to certain carriers through hydrogen bonds. These are weaker than covalent (permanent) bonds, but still strong enough to hold a fragrance molecule close. Think of it like Velcro: easily attached, easily pulled apart. When you add heat, moisture, or an acidic environment, the fragrance is released bit by bit, delivering a more drawn-out scent experience.
1.2 Physical Encapsulation (“Capturing”)
Macrocyclic Musks
Picture a large, ring-shaped molecule that can act a bit like a pocket, welcoming smaller, more volatile fragrance molecules inside. This “host-guest” effect—driven by hydrophobic (water-repelling) interactions—creates a cozy nest so your perfume’s precious top notes don’t flee at the first sign of fresh air.
Well-known macrocyclic musks include ambrettolide, civetone, and muscone—which themselves have musky, warm nuances that deepen a fragrance while helping to slow down overall evaporation.
Supramolecular Gels
In modern perfumery, low-molecular-weight (LMW) gels or other polymeric carriers can physically lock fragrance components in a mesh of fibers. It’s much like capturing raindrops in a sponge, then letting them drip out slowly. This technique shows great promise for products like body lotions, fabric care, and even home fragrances.
2. Types of Fixatives You’ll Encounter
When we talk about “fixatives,” we’re often referring to large or heavy-scent molecules that anchor a blend. Some examples:
Schiff Bases
Aurantiol (Schiff 001): Formed from hydroxycitronellal and methyl anthranilate. Slows aldehyde evaporation through a reversible bond.
Macrocyclic Musks
Ambrettolide, Civetone, Muscone, Ethylene Brassylate: Large, ring-shaped molecules offering a smooth, musky base. They’re highly sought for their ability to provide both softness and staying power.
Polycyclic Musks
Galaxolide (HHCB) and Celestolide: More contemporary synthetic musk molecules that add volume and longevity, though they don’t always form strong “hosts” like macrocyclics.
Nitro Musks
Musk Xylene, Musk Ketone: Once very popular, they’re now less common due to regulatory considerations. Still recognized for their intense and long-lasting muskiness.
Resins & Balsams
Labdanum, Benzoin, Peru Balsam: Natural, sticky exudates from plants. They may not form chemical bonds with your perfume’s fleeting top notes, but their heavy, resinous nature “weighs down” a blend, slowing overall evaporation.
3. Factors That Affect Fixation
Beyond the raw materials themselves, several outside influences decide how effectively a fragrance will “stick around”:
Polarity & Functional Groups
Aldehydes and ketones often participate in hydrogen bonding or can form Schiff bases if an amine is present. Meanwhile, purely hydrocarbon-based materials might need a different approach to remain anchored.
pH Levels & Moisture
Many of these bonding systems are sensitive to acidity or humidity. Add water or lower the pH, and a Schiff base may break down, or a hydrogen bond might be released, leading to a sudden burst of scent.
Solvent Choice
Ethanol is an industry favorite for good reasons—it evaporates quickly, leaving behind the fragrance. But silicone carriers, diethyl phthalate, or other specialized solvents may alter how quickly or slowly your perfume’s fixatives go airborne.
Environmental Conditions
Temperature, airflow, and even your skin’s moisture all influence how a fixative behaves. Warm, humid days can accelerate diffusion of certain scent molecules, while cooler, drier conditions slow it down.
4. Putting It All Together
If you’ve ever noticed a perfume that seems to have a “second wind” hours later, you’ve witnessed a fixative at work. In some cases, it’s a Schiff base quietly releasing aldehydes; in others, a musky macrocycle is gently cradling a floral note. Resins, musks, and specialized gels can all contribute to the same outcome: a fragrance that endures and evolves gracefully on your skin.
From a perfumer’s perspective, layering these strategies—chemical bonding, physical capture, heavy resins—can yield a more sophisticated, three-dimensional fragrance. For fragrance enthusiasts, understanding these techniques helps demystify why some perfumes carry you through your day, while others fade away by lunchtime.
Conclusion
Perfume fixation is as much an art as it is a science. Whether via clever chemical bonding (true fixation), gentle physical trapping, or simply using robust ingredients with low volatility (complementary fixation), the goal remains the same: to keep a perfume alive and delightful for as long as possible.
Modern research, including the use of low-molecular-weight gels, specialized silicone carriers, and newly designed profragrances, continues to expand our toolkit. The next time you’re savoring a fragrance’s lingering dry-down, remember: there’s real chemistry—and a dash of magic—in every breath.
For an in-depth analysis of the physical chemistry, thermodynamics, and molecular mechanisms underlying fixative function, read: Molecular Mechanisms of Perfume Fixatives: A Physical Chemistry Perspective.





Pingback: Molecular Mechanisms of Perfume Fixatives: A Physical Chemistry Perspective - olfactive aesthetics author's niche perfumery
October 18, 2025
Franziska
January 1, 2026
Thank you very much for this great insight! It is very rare to find such qualified infos about scent creation! Please inform me, when you post new articles!