Raw Materials in Perfumery That Prevent Oxidation
Oxidation in perfumery is a major concern because it can lead to the formation of various chemical species such as peroxides, organic hydroperoxides, peroxyhemiacetals, hemiacetals, acetals, or transesterification products. These substances may alter the organoleptic properties or appearance of a perfume ingredient, a formulated perfume, a body care product, an essential oil, or a natural extract. In some cases, they can even be harmful, irritating, or allergenic. Most importantly for perfumers, oxidation also substantially impacts a perfume’s aroma profile, diminishing the fragrance’s intended character and overall quality.
One frequent outcome of oxidation is the rancidity of oils. For example, Davana oil can turn rancid, shifting its fragrant profile to a hay- or fenugreek-like scent. Similar changes occur in other natural oils, which can develop a flat or distinctly sour note over time. Moreover, excessive limonene in citrus oils can be oxidized to p-cymene, which possesses a sour and “flat” character rather than the bright, zesty nuance of fresh limonene. Phenyl Ethyl Alcohol may also be oxidized first into Phenylacetaldehyde, then further into Phenylacetic Acid—altering its initial floral sweetness into a sharper, acidic aroma.
In certain natural extracts containing unsaturated fatty acids, oxidation can spur polymerization, creating sediment. This not only affects the scent but also detracts from the product’s aesthetic appeal. To counteract these issues, perfumers and formulators often introduce specific additives or compounds that are more easily oxidized than the key fragrance materials, effectively protecting the core scent from damage.
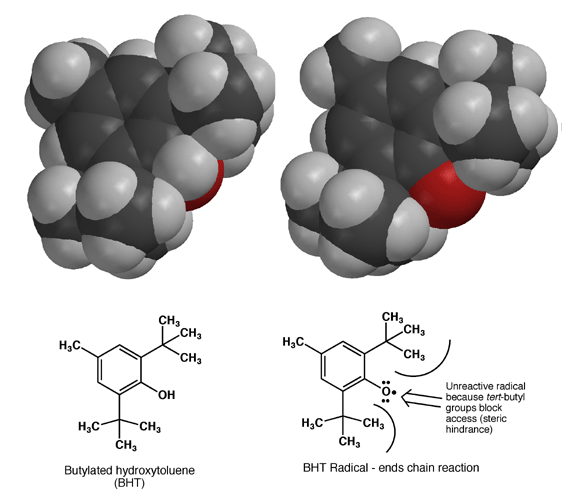
A commonly used antioxidant in perfumery is BHT (Butylated Hydroxy Toluene), frequently combined with UV protectors like benzophenone to slow down oxidation. BHT’s strength lies in its ability to form a stable radical on its oxygen atom, thanks to the resonance stabilization afforded by its benzene ring. Once the BHT radical forms, large tert-butyl groups create steric hindrance, preventing further reactions and effectively halting the chain reaction of oxidation. This safeguards the remaining fragrance components from oxidative damage.
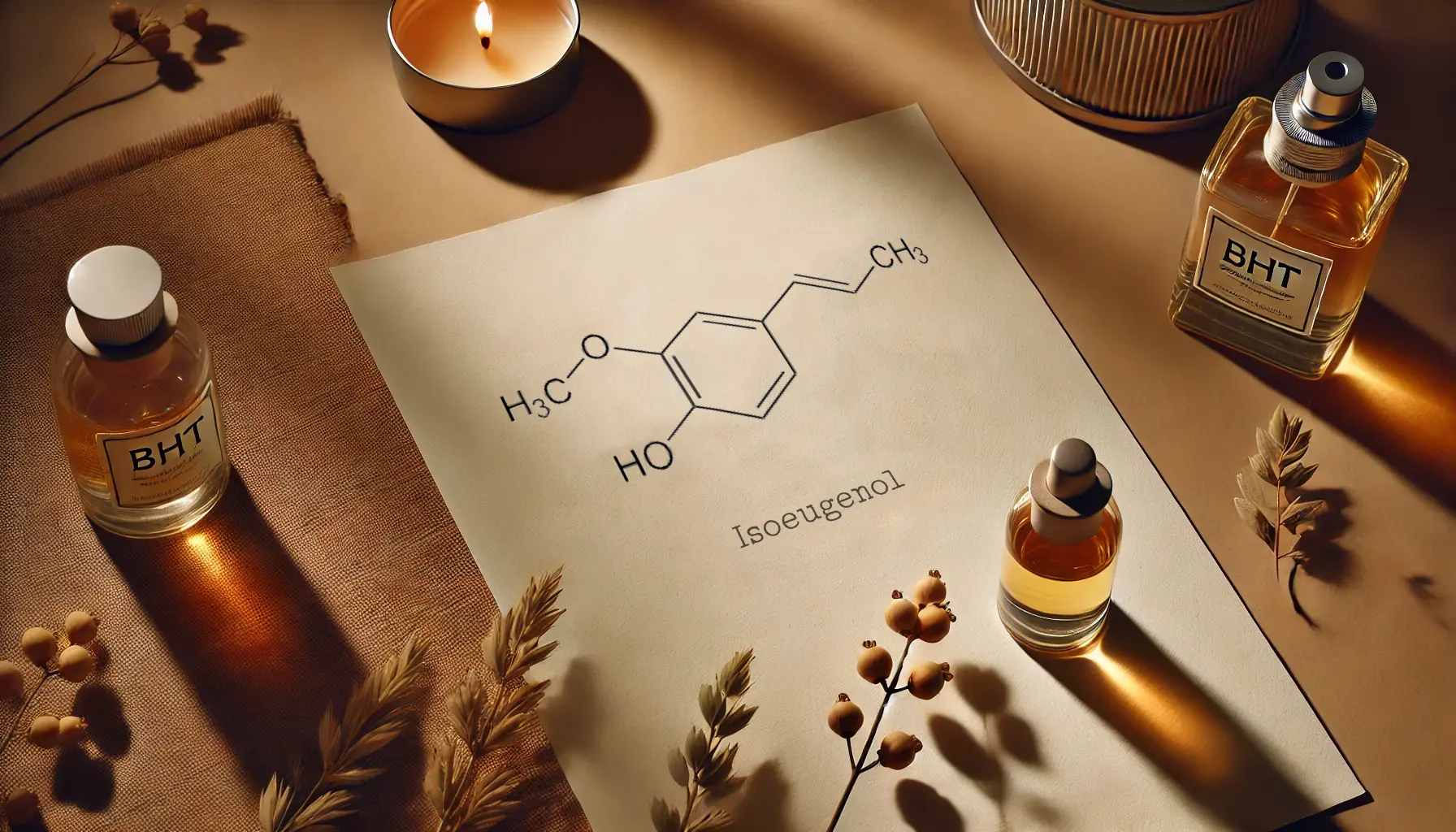
Isoeugenol also stands out for its antioxidant qualities. It is a natural compound found in perfumes, soaps, detergents, air fresheners, and used as a flavoring agent in cosmetics and food products. Known for its antioxidant and anti-inflammatory properties, isoeugenol has been further modified via Friedel–Crafts alkylation, yielding derivatives shown to outperform standard antioxidants like BHT, BHA, and Trolox in some studies. Since isoeugenol can be synthesized from eugenol through isomerization, it represents an accessible option for creating novel antioxidant solutions in perfumery.
From rancidity control to preserving the desired scent profile, these measures are essential for maintaining product integrity and longevity. Whether relying on BHT, isoeugenol derivatives, or other cutting-edge stabilizing agents, ensuring effective oxidation prevention is a cornerstone of modern perfumery. By selecting the right Raw Materials and antioxidant strategies, perfumers can deliver fragrances that remain true to their original formulation, delighting consumers with each experience.





Pingback: Aging and Maturation in Natural Perfumery - olfactive aesthetics author's niche perfumery
February 3, 2025